July 12, 2017
Seema Verma, Administrator
Centers for Medicare & Medicaid Services
7500 Security Blvd.
Baltimore, MD 21244
Attention: CMS-9928-NC
Dear Administrator Verma
Thank you for the opportunity to respond to your request for information on reducing regulatory burdens and improving coverage options under the Patient Protection and Affordable Care Act (ACA) Title I.
The National Committee for Quality Assurance (NCQA) is a non-profit that for 27 years has worked to improve the quality and value of health care through measurement, transparency and accountability.
- We are the United States’ largest health plan accreditor, and accredit a large majority of ACA plans. More than 169 million Americans are in plans that have earned NCQA Accreditation by demonstrating that they follow evidence-based processes and deliver high-quality care.
- We steward the Healthcare Effectiveness Data and Information Set (HEDIS®)[1], the most widely used set of clinical quality measures. 175 million Americans are in plans that report HEDIS – over 80% of all health plan enrollees.
- We also have the nation’s largest Patient-Centered Medical Home program, with nearly 1 in 5 primary care clinicians in NCQA-Recognized PCMH practices, and the nation’s only Patient-Centered Specialty Practice program that builds on PCMHs to create “medical neighborhoods.”
Our comments focus on reducing state burden in plan oversight by building on and strengthening the Medicare Advantage and Medicaid deeming programs. We also urge you to reduce the burden of reporting clinical quality measures that the ACA requires for Exchange plans by moving to well-designed & tested electronical clinical quality measures.
Deeming Accredited Plans
The ACA requires Exchange plans to have the performance-based accreditation that NCQA provides. Because states running Exchanges must oversee these plans, you could reduce oversight burdens by letting states deem accredited plans as meeting ACA requirements. This parallels what CMS now allows in deeming for both Medicare Advantage and Medicaid managed care plans.
Deeming accredited plans reduces burden on regulators and plans by eliminating duplicative reporting requirements, while ensuring that plans meet rigorous, evidence-based quality standards. Our program drives quality improvement by scoring insurers on both clinical quality performance and patient experience measures. NCQA-Accredited plans consistently outperform non-accredited plans on key quality measures like access, control of blood pressure, cholesterol and diabetic blood sugar, and breast and colorectal cancer screening.
Because of the value that accreditation and deeming provide, 25 states exclusively require NCQA accreditation for Medicaid managed care plans, another 5 states require accreditation and accept NCQA as meeting the requirement, and 16 states give NCQA-Accredited plans credit for meeting some Medicaid regulations such as utilization management. Each year we update our Medicaid Managed Care Toolkit that explains how states can take advantage of the federal authority to streamline oversight of Medicaid managed care plans through private accreditation.
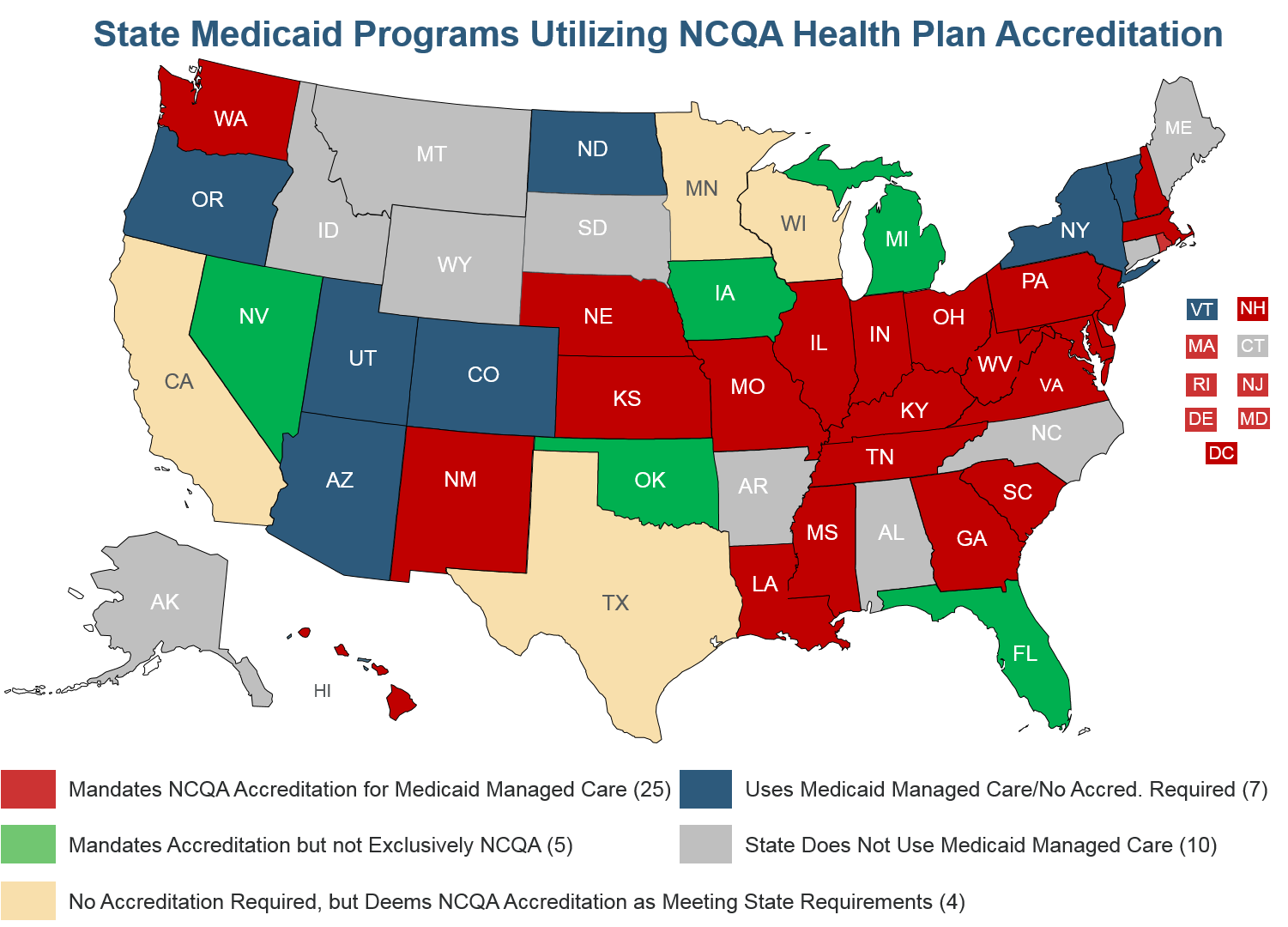
CMS deemed NCQA-accredited Medicare Advantage plans from 2000-2014, and we have been working constructively with your staff to revive and strengthen the program. Deeming of accredited plans streamlined CMS oversight while providing both rigorous accountability and regulatory relief for plans.
Our work to revive the program is hampered, however, by federal law which authorizes Medicare Advantage deeming in just six categories: Access, Advanced Directives, Anti-discrimination, Confidentiality & Accuracy, Provider Participation (credentialing) and Quality Assurance. The previous administration suspended MA deeming in 2015 because its plan audits focused primarily on access and not on other important deemable categories.
As a result, there was little oversight of other deemable categories, minimal overlap between CMS audits and NCQA’s deeming program, and little tangible value for either CMS or plans.
Working with CMS, we have made some progress towards reviving our deeming program. We are exploring whether our Managed Long Term Services and Supports Accreditation could be adapted to deem PACE and Medicare-Medicaid Financial Alignment plans. We discussed the potential for us to verify that networks do not include HHS Inspector General-excluded providers. We also discussed whether legislative or regulatory changes could help to revitalize deeming. This is all very promising.
However, we have not yet identified enough substance to add into our deeming program to incentivize plans to pay for deeming. We are eager to work with you to find the best course of action so that deeming can once again provide meaningful value and burden relief for both CMS and plans.
In conversations with several key Hill and Administration offices, we have found bipartisan support for tweaking the legislation to authorize deeming in “other categories (HHS) Secretary considers appropriate,” beyond the six categories listed in the statue now. This would allow CMS to tailor deeming to track oversight priorities over time, and maximize private accreditation’s potential to reduce oversight burden while ensuring rigorous oversight and evidence-based standards. It also provides a template for a high-quality deeming program for Exchange plans.
Reducing Reporting Burden with eCQMs
The ACA, in §1311(c)(1)(D), requires Exchange plan accreditation based on “clinical quality measures such as” HEDIS. To reduce the burden involved in reporting quality measures, NCQA urges the Administration to drive the implementation of well-designed and properly tested electronical clinical quality measures (eCQMs). Claims-based measures lack data on outcomes and often reflect care provided long in the past.
However, using validated electronic clinical data systems can support innovative outcome measures – a key objective for health reform today. Even more importantly, these measures could be derived from data entered into electronic systems as a natural part of clinical workflow.
Leveraging this validated data is critical to eliminating much of the burden involved in quality reporting. As public and private payers increasingly pay clinicians based on the quality of care they provide, it will become ever more important that the quality data they receive is accurate and error-free. NCQA’s eMeasure Certification is a solution to this challenge.
Our program evaluates the accuracy of electronic systems that calculate eCQMs by validating the software logic used to produce measure results. We use consistent data files to streamline what is normally a resource-intensive mapping process. Our program validates accuracy when supplemental data is used for quality reporting, thereby removing altogether the primary source audit burden. Using exclusively electronic data also decreases or eliminates entirely the burden and cost of chart review. Organizations that rely on eMeasure data can trust that NCQA-certified systems have gone through the industry’s most rigorous assessment and validation of data accuracy.
But most importantly, we can certify the software that third-party data aggregators (such as physician registries) use for quality reporting. These aggregators collect data directly from EHRs to report quality measures and we can certify the accuracy of those measure results.
We also can work directly with those aggregators to ensure that measure specifications stay up-to-date, eliminating the need to update specifications in thousands of individual EHR installations.
We strive to reduce quality measurement burden by working upstream of the physician. We want to take work required for quality reporting out of physicians’ hands and give them more time to focus on their patients. We can do this by leveraging more electronic data to produce quality measures, but we also must validate the accuracy of those results. NCQA is eager to work with you to achieve this goal.
Thank you for the opportunity to comment. Please contact Paul Cotton, Director of Federal Affairs, at 202 955 5162 or cotton@ncqa.org if you have any questions.
Sincerely,
Margaret O’Kane,
President
[1] HEDIS is a registered trademark of NCQA.
