Data Aggregator Validation
Validate Clinical Data Streams for Use in Reporting and Other Quality Programs
The Data Aggregator Validation program evaluates an organization’s management and exchange of health data. Data streams that earn validation undergo a rigorous, end-to-end look at the quality and integrity of data and the procedures used to manage and safeguard it. From ingestion at primary sources through transmission to end users, verifies adherence to NCQA process, system and data standards.
Earning the Data Aggregator Validation designation conveys to health plans, providers, government organizations and others that they can trust the accuracy of aggregated clinical data for use in Healthcare Effectiveness Data and Information Set (HEDIS®) reporting and other quality programs. NCQA is trusted industry-wide for setting standards and creating programs that improve health care quality.
Read about how the Data Aggregator Validation program impacts health plans and the HEDIS Compliance Audit.
Benefits
Trust data accuracy
NCQA’s Data Aggregator Validation program evaluates the ingestion, transformation and output of clinical data to help ensure data integrity.
Showcase safe data management
During validation, organizations undergo a rigorous, end-to-end look at the quality and integrity of data and the procedures used to manage and safeguard it.
Improve efficiencies
Data from health information exchanges (HIEs) and other data aggregators are increasingly used as supplemental data for HEDIS reporting.
Support contracting
Contracting partners are looking for assurances that data they ingest is accurate. A validation from NCQA helps give your contracting partners more peace of mind and makes your data more valuable.
Serve as an industry leader
Demonstrate to payers and partners that your organization has best-in-class data management practices and is taking steps to improve interoperability in the industry.
Trust data accuracy
NCQA’s Data Aggregator Validation program evaluates the ingestion, transformation and output of clinical data to help ensure data integrity.
Showcase safe data management
During validation, organizations undergo a rigorous, end-to-end look at the quality and integrity of data and the procedures used to manage and safeguard it.
Work with a data partner
Data from health information exchanges (HIEs) and other data aggregators are increasingly used as supplemental data for HEDIS reporting. Data from data streams validated by NCQA can be used as standard supplemental data in HEDIS reporting, eliminating the need for primary source verification (PSV) during the HEDIS audit process.
This saves time and money for aggregators, provider organizations and health plans. Read about how the Data Aggregator Validation program impacts health plans and the HEDIS Compliance Audit.
Support contracting
Contracting partners are looking for assurances that data they ingest is accurate. A validation from NCQA helps give your contracting partners more peace of mind and makes your data more valuable.
Serve as an industry leader
Demonstrate to payers and partners that your organization has best-in-class data management practices and is taking steps to improve interoperability in the industry.
Eligible organizations
Any data stream that meets the standards of the program can be validated by NCQA. The validated status is conferred to the data stream. Organizations that are responsible for certain standards as part of the validation will earn a Certified Data Partner status for Data Aggregator Validation and can advertise themselves as supporting the NCQA Data Aggregator Validation program. There are two statuses that successful participates can achieve.
Responsible Party
Organizations that perform all functions in the program requirements, including facilitating primary source verification, can act as the sole responsible party for meeting validating a data stream by meeting all program standards.
Responsible Party
Organizations that perform all functions in the program requirements, including facilitating primary source verification, can act as the sole responsible party for meeting validating a data stream by meeting all program standards.
Data Partner
The aggregation of data sometimes involves multiple parties. For example, an organization might be responsible for the management of the data and output of the CCD or FHIR® file, but not have legal access to the primary source data to support PSV. In these cases, the organization that can facilitate PSV is the responsible party for the data stream, but they can partner with other organizations to validate a data stream.
Data Partner
The aggregation of data sometimes involves multiple parties. For example, an organization might be responsible for the management of the data and output of the CCD or FHIR® file, but not have legal access to the primary source data to support PSV. In these cases, the organization that can facilitate PSV is the responsible party for the data stream, but they can partner with other organizations to validate a data stream.
The Program Journey – Now offering FHIR®
The first step to validating a data stream is a discussion with an NCQA program expert. Purchase and review the program resources and conduct a gap analysis against the program requirements to ensure you will meet the program requirements. Once ready, submit your online application.
Align your internal processes and policies with the three primary validation activities of the Data Aggregator Validation requirements.
- Process standards review: These standards focus on how the data are managed throughout the process. Requirements look at things like how the data are ingested, processes for managing the validating data, coding integrity, quality assurance and change management, governance and data security.
- Primary Source Verification (PSV): The PSV process verifies that the information in the final CCD or FHIR® file matches the actual original primary source data.
- Conformance to the NCQA guidelines: Output CCD or FHIR® files must conform with the CCD implementation guide. Validation can take anywhere from 12-18 weeks depending on the number of clusters and complexity of the validation.
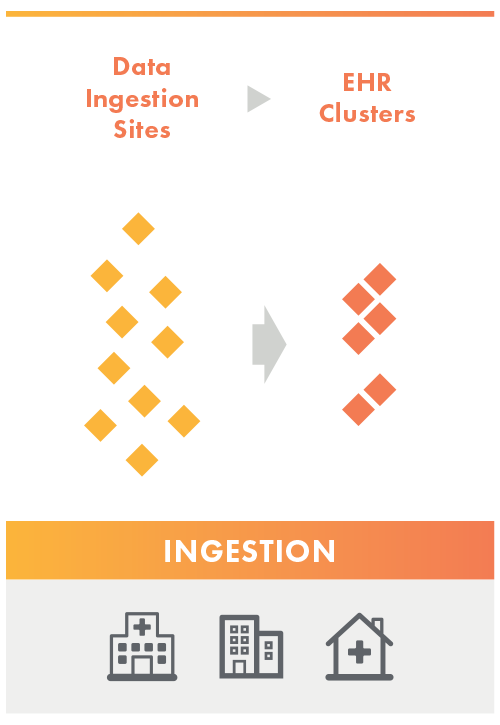
Ingestion
The Data Aggregator Validation customer completes an inventory of the ingestion sites they would like to get validated on the Data Submission Log (DSL). The validator reviews the inventory list and groups ingestion sites into clusters based on like care setting + EHR type.
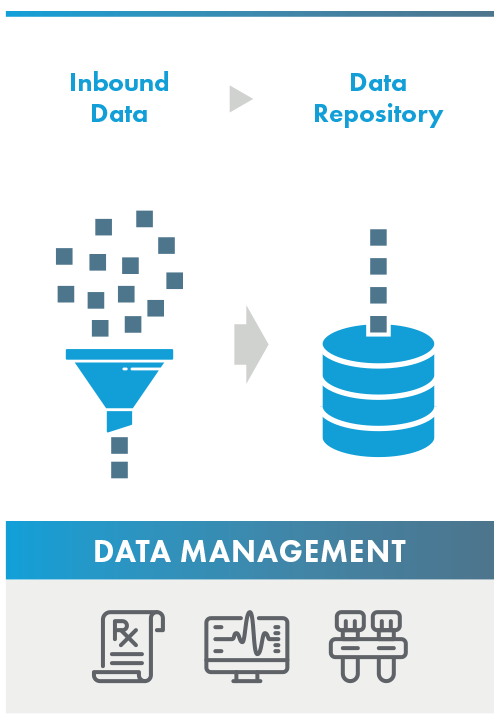
Data Management
The validator analyzes the responsible parties’ process, system, and data standards from the point the data is inbounded through when the data is added to the responsible parties’ data repository. While a data partner does not have legal access and cannot support Primary Source Verification, a Data Partner may support a Responsible Party by meeting one or more program standard.
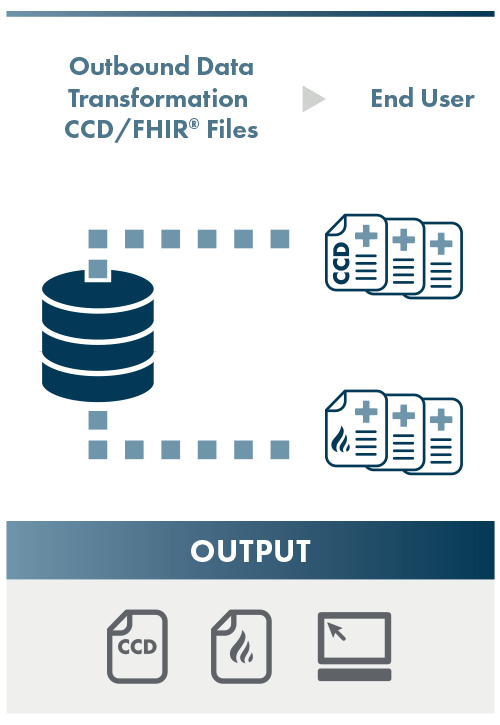
Output
NCQA assesses the conformance of the outbound CCD or FHIR® data to the specified HL7 CCD or FHIR® Implementation Guide. The primary source verification process involves ensuring that the data in the conformed output CCD or FHIR® resources matches the primary source data found in the source EHR.
Offering Both CCD and FHIR® Options
Continuity of Care Documentation (CCD) and Fast Healthcare Interoperability Resources (FHIR) are both healthcare industry standards for exchanging patient health information, with CCD being the more established.
CCD, in use since the mid-2000s, was developed as a part of the Health Level Seven International (HL7) standards, specifically HL7 CDA (Clinical Document Architecture) standard.
FHIR®, introduced in 2011, and developed by HL7, is a more recent standard designed to improve healthcare data exchange and interoperability.
Several key policies and initiatives, including the 21st Century Cures Act, the ONC Cures Act Final Rule, and the CMS Interoperability and Patient Access Rule, actively endorse and promote the further adoption of FHIR® as the standard of choice for healthcare data exchange.
NCQA supports organizations at every stage of their data quality journey. Connect with a representative today to see which option is the best fit for your organization.
NCQA works with organizations to ensure their data output adheres to the HL7 C-CDA R2.1 Implementation Guide in the June 2020 errata package, available on the HL7 C-CDA product page, or in the US Core Implementation Guide STU3 (v3.1.1 R4) available in the HL7 FHIR Implementation Guide Registry.
See Validated Organizations
View the organizations participating in NCQA’s Data Aggregator Validation program and see the report cards.

Data Aggregator Validation
NCQA’s Data Aggregator Validation Program evaluates clinical data streams to help ensure that health plans, providers, government organizations and others can trust the accuracy of aggregated clinical data.
EVALUATION PRODUCT
Data Aggregator Validation
STATUS
Validated Data Stream
NEXT REVIEW DATE
06/02/2024

