January 9, 2017
Seema Verma, Administrator
Center for Medicare & Medicaid Services
7500 Security Blvd.
Baltimore, MD 21244
Attention: CMS-4182-P
Dear Administrator Verma:
Thank you for the opportunity to comment on proposed Medicare Advantage and related program changes for 2019. The National Committee for Quality Assurance (NCQA) strongly supports letting plans limit opioid prescriptions to specific prescribers and pharmacies for at-risk beneficiaries. This closely parallels our new HEDIS®[1] opioid measures that track high dosages and multiple prescribers. We urge you to add these and other behavioral health measures[2] to the Medicare Advantage Stars to emphasize their importance with the powerful Stars bonus and rebate financial incentives.
We also support letting plans reduce cost sharing for certain benefits, tailor supplemental benefits, and vary deductibles for patients with specific medical criteria. Experience with such value-based insurance design in employer plans shows that it can increase use of high-value treatments and providers in ways that lower overall costs and disease burden over time.
We support adding anti-fraud efforts and Medication Therapy Management (MTM) program costs to the medical loss ratio. Both improve quality and should count as other quality efforts do. We do not support, however, letting plans merely report medical loss ratio percentages rather than specific details, as that would limit the ability to track spending on quality improvement and assess its impact.
We further support changing policy on Star ratings when plans consolidate. The proposal to base ratings on enrollment-weighted scores is better than allowing plans to apply a higher rating from a remaining contract to consolidated lower-rated plans in other states. However, it would be much better to issue contract numbers and base ratings at the state level. This would provide a more accurate assessment of actual plan quality that beneficiaries need to make truly informed decisions.
We also have concern about ending the “meaningful difference” requirement for plans offered by the same issuer. This could lead to “choice overload” that makes it difficult for beneficiaries to choose the best plan. It would not encourage innovation, as suggested, since true innovation by definition should provide a meaningful difference.
Detailed comments on these and other issues in the proposed rule are below.
Opioid Limits: We strongly support the proposal to let plans limit opioids to certain prescribers and/or pharmacies for enrollees at risk of addiction. This is an urgently needed policy that can help to address the opioid epidemic sweeping our nation. It also parallels our new HEDIS opioid measures:
- Use of Opioids at High Dosage. This measure assesses the rate of health plan members 18 years and older who receive long-term opioids at high dosage (average morphine equivalent dose >120mg) for 90 consecutive days or longer.
- Use of Opioids from Multiple Providers. This measure assesses the rate of health plan members 18 years and older who receive opioids from 4 or more prescribers and 4 or more pharmacies.
High dosages, along with multiple prescribers and pharmacies, are all risk factors for dangerous overdose and death. Our intent is for these measures to encourage health plans to address the opioid epidemic and track their progress.
These measures are among a growing suite of behavioral measures we have or are now developing because behavioral health conditions are substantially undertreated and strongly associated with increased physical health need. We also have measures addressing the many behavioral and mental health treatments that have significant side effects that require careful monitoring and treatment. We include a list of all these measures in Appendix A. We urge you to add these measures to the Star Ratings to make improving behavioral and mental health care a Medicare Advantage priority.
Value-Based Insurance Design (VBID): We support allowing plans to reduce cost sharing for certain benefits, tailor supplemental benefits, and vary deductibles for patients with specific medical criteria. Experience with such value-based insurance design in employer plans shows that it can increase use of high-value treatments and providers in ways that lower overall costs and disease burden over time.
Reduced cost sharing for highly effective preventive services and chronic care therapies addresses financial barriers that can prevent people from getting needed care and improves adherence to high-value treatments. This in turn can prevent costly complications and yield net savings.
There is already substantial evidence on the powerful benefits of VBID.
- Private employers like Pitney Bowes have found significant savings from reduced cost sharing for essential diabetes treatments.
- The Oregon Public Employee’s and Educators Benefit Board’s extensive VBID program improved HEDIS scores, increased use of preventive services, and provided substantial savings.
- The U.S. Department of Health & Human Services prominently features VBID in its National Quality Strategy.
- And the University of Michigan Center for Value-Based Insurance Design tracks case studies and more at http://www.sph.umich.edu/vbidcenter.
This provides more than sufficient evidence to incorporate VBID throughout the Medicare Advantage program as proposed while the Medicare Advantage-specific VBID demonstration program progresses and develops further evidence on its efficacy.
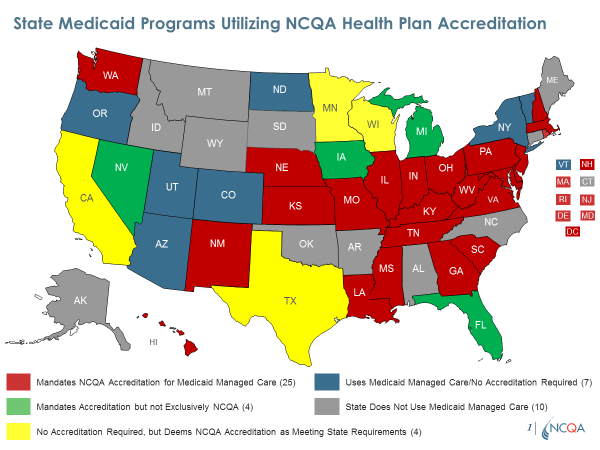
Default Enrollments: To ensure quality when allowing passive enrollment into Medicaid plans after non-renewal of a plan serving people dually eligible for Medicare and Medicaid, we urge you to require NCQA performance-based Accreditation. We publicly report on the quality of all NCQA-Accredited plans including Medicaid plans. Most states require or recognize NCQA Accreditation for their Medicaid plans. We also have the only program accrediting plans providing long-term services and supports (LTSS), which some states require in Medicare-Medicaid Financial Alignment demonstrations. These features of NCQA’s Medicaid Accreditation program provide the detailed assessment of quality needed for determining which plans should qualify for default enrollments. NCQA-Accredited plans also perform demonstrably better on key quality indicators such as Medicare Star Ratings.
Medical Loss Ratio: We support adding anti-fraud efforts and Medication Therapy Management (MTM) program costs to what counts as medical spending for the medical loss ratio. Both anti-fraud efforts and MTM programs help to improve quality and should count, as other quality efforts already do. Fraud, for example, can include claims for needed care not delivered and delivery of unnecessary care, both of which have potential to cause physical as well as financial harm to enrollees. MTM programs focus on appropriate drug utilization among patients with complex care needs, for whom inappropriate utilization can be common and especially problematic.
We do not support, however, letting plans merely report medical loss ratio percentages rather than specific details, such as quality improvement activity spending. Doing so would limit the ability to track spending on quality improvement and assess the degree to which the size and type of specific efforts result in better outcomes. If you do let plans simply report percentages, we urge you to conduct random audits to guard against the significant potential for gaming.
Open Enrollment: We appreciate the concern for consumers suggested by the proposal to let beneficiaries make an additional plan switch as late as March 31, rather than February 14 as under current policy. We note, however, that doing so will impact Medicare Advantage Star Ratings, as many measures require more than 9 months of continual enrollment. Enrollees who switch as late as March 31 will not be eligible for inclusion in denominators for several measures. While the number of enrollees who make such changes will likely be small, one result will be a less robust picture of quality.
Improving Measures: Regarding your inquiry on how to improve measures to better reflect the quality of outcomes, we urge you to develop measures and systems to reward plans for reducing disparities in care. Racial, ethnic and other socioeconomic disparities persist throughout our healthcare system. However, our own internal analysis shows that Medicare Advantage plans with high levels of low-income enrollees nonetheless are able to achieve high quality in all parts of the country.[3] It is therefore clearly possible to reduce and ultimately eliminate disparities in Medicare Advantage, and to rate each Medicare Advantage plan on disparities. Doing so would provide a powerful incentive for all plans to achieve the low rates of disparities that we found in our analysis.
To improve Star Ratings overall, you could reward plans for reporting via electronic clinical data systems, such as provider electronic health record data. Electronic health data would allow for measurement of meaningful outcomes that we cannot assess with claims data or without burdensome medical record review. CMS established precedent for this in Medicare Access & CHIP Reauthorization Act Merit-based Incentive Payment System regulations providing end-to-end electronic reporting bonus points. Electronic reporting can enhance the ability to audit and ensure accuracy.
Geographic/Market Area Characteristics: We are skeptical about potentially adjusting Star Ratings for geographic factors such as serving areas that are rural or dominated by provider monopolies. Doing so could dis-incentivize plans from striving to provide the highest quality in all areas they serve. Any such adjustments, if made, should be to payments, as CMS is doing for plans with enrollees who are dually eligible and/or disabled. You should not adjust Star Ratings directly, as that would merely mask without actually addressing any related quality issues.
New Entrants: To level the field on quality between new and experienced Medicare Advantage plans, you could temporarily enhance new entrants’ scores based on NCQA performance-based Accreditation.
NCQA-Accredited plans perform better on many Stars measures, including measures of prevention, appropriate treatment and patient experience.[4] Therefore NCQA Accreditation is a strong proxy for high quality that you could rely on for new plans.
Physician Experience: We believe you should measure and reward plans for the experience they provide to physicians and other clinicians, as plan-clinician relationships are a critical factor in maintaining and improving quality. Clinician experience measures could, for example, assess the frequency and substance of feedback from plans to providers on gaps in care, resource use, and other quality issues on which plans and clinicians achieve the best results when working together as partners.
Contract Consolidations: We, like the Medicare Payment Advisory Commission (MedPAC) and others, believe it is urgently important to change current policy that lets plans apply a higher rating from a remaining contract to lower-rated plans in other states when issuers consolidate contracts. However, using enrollment-weighted averages of scores among consolidating plans would still give lower-rated parts of consolidated contracts higher scores than they have earned. This would mislead enrollees and unfairly skew quality-based bonuses and rebates intended to drive enrollees to genuinely higher-rated offerings.
A better approach would be to issue contract numbers and base Star ratings at the state level. This would provide a more accurate quality assessment to help beneficiaries make informed choices and better ensure that quality bonuses and rebates drive enrollment to the highest quality offerings.
Adding, Updating & Removing Measures: We appreciate interest in moving measure updates from sub-regulatory methods to the Federal Register, and related changes in the timeline for changing measures. We also agree it is critically important to continue allowing some sub-regulatory changes, such as for updated clinical guidelines or reliability issues. However, you should not put measures on the Display Page for two years when measure stewards decide to update rather than remove them. This will lead to unnecessary gaps in measurement for critically important issues for which plans need to be accountable.
We recommend moving the Plan All-Cause Readmission measure to the display page for measurement year 2019 due to significant changes we are considering. We plan to publish these changes in 2018 and then to apply them in measurement year 2019.
Additional measures for which we plan revisions in the next two years include:
- Care for Older Adults Medication Review, Functional Status Assessment and Pain Assessment: We are developing a new measure – Health Assessment for People with Multiple High-Risk Chronic Conditions – that would potentially replace the Care for Older Adults measure for HEDIS 2020 (measurement year 2019). If approved, we plan to publish these changes in 2018 and to apply them in measurement year 2019 to all Medicare Advantage Plans.
- Controlling Blood Pressure: We are updating this measure based on new guidelines published in late 2017 and the changes could be substantial. If approved, we plan to publish the changes in 2018 as part of the HEDIS 2019 publication.
- Osteoporosis Management in Women who had a Fracture: We are planning to re-evaluate this measure for potentially substantial revisions that we would publish in 2019.
- Rheumatoid Arthritis Management: We are planning to re-evaluate this measure for potential revisions or retirement that we also would publish in 2019.
- Medication Reconciliation Post-Discharge: We plan to retire this measure pending first-year analysis of an identical indicator in the Transitions of Care measure, which should replace it.
- Risk of Chronic Opioid Use: We are developing this measure for HEDIS 2020 (measurement year 2019). If approved, we will publish it in 2019 and it would apply to all Medicare Advantage Plans.
- We also are developing a measure specified for electronic reporting to assess receipt of recommended adult immunizations. It will incorporate Pneumococcal Vaccination Coverage for Older Adults, which would cease to be a stand-alone measure.
Patient Experience, Complaints & Access Measures: We appreciate your interest in potentially increasing the weight for such measures from the current 1.5 to 3. These measures are critically important and could benefit from greater attention from plans that increased weighting generates. However, we encourage you to not move forward on this until we have better measures in these areas. The Consumer Assessment of Health Plans Survey (CAHPS) is long and has low responses and long lags in feedback that does not target which enrollees are having what types of problems. Complaint measures also merely assess raw numbers and do not yet differentiate between low-level issues, multiple calls from the same enrollee, and other important distinctions. Once we have better measures in these areas, it would make sense to consider increasing the weights.
Meaningful Difference: We do not support the proposal to repeal the “meaningful difference” requirement for plans offered by the same issuer. Allowing issuers to offer plans in the same locations that do not provide substantially different benefit packages could lead to “choice overload” and make it difficult for beneficiaries to choose the best plan. Removing the meaningful difference requirement will not enhance competition, as choices lacking meaningful difference only foster confusion. It also will not encourage innovation, since true innovation by definition should provide a meaningful difference.
Thank you again for inviting our comments. If you have questions, please contact Paul Cotton, Director of Federal Affairs, at cotton@ncqa.org or (202) 955-5162.
Sincerely,
Margaret O’Kane,
President
Appendix A: Healthcare Effectiveness Data and Information Set (HEDIS) Mental Health & Behavioral Measures
| Domain | Measure | Status | Data Source | Denominator | Numerator |
| Screening | Depression screening and follow up | In HEDIS | Electronic Clinical Data Source (ECDS) | Members 12 years of age and older. | Members who were screened for clinical depression using a standardized tool and received appropriate follow-up care if screened positive. |
| Alcohol screening and brief intervention | In HEDIS | ECDS | Members 18 years of age and older. | Members who had a systematic screening for unhealthy alcohol use and received brief intervention if screened positive. | |
| Symptom Monitoring | Utilization of the PHQ-9 to Monitor Depression Symptoms for Adolescents and Adults | In HEDIS | ECDS | Members 12 years of age and older with a diagnosis of major depression or dysthymia. | Members who had a PHQ-9 tool administered at least once during a four-month period. |
| Medication adherence | Adherence to antipsychotic medications for individuals with schizophrenia | In HEDIS | Claims | Members 19–64 years of age with schizophrenia.
| Members who achieved a PDC of at least 80% for their antipsychotic medications. |
| Antidepressant medication management | In HEDIS | Claims | Members 18 years of age and older who were treated with antidepressant medication and had a diagnosis of major depression. | · Initiation Phase. Members who had at least 84 days of continuous treatment with antidepressant medication beginning on the index prescription start date (IPSD) through 114 days after the IPSD. · Continuation Phase. Members who had at least 180 days of continuous treatment with antidepressant medication beginning on the IPSD through 231 days after the IPSD. | |
| Medication management | Follow-up care for children prescribed ADHD medication | In HEDIS | Claims | Children 6–12 years of age who were newly prescribed ADHD medication. | · Initiation Phase. Members who had an outpatient, intensive outpatient or partial hospitalization follow-up visit with a practitioner with prescribing authority within 30 days after the IPSD. · Continuation and Maintenance Phase. Members with a prescription dispensed for ADHD medication, who remained on the medication for at least 210 days and who, in addition to the visit in the Initiation Phase, had at least two follow-up visits with a practitioner within 270 days after the Initiation Phase ended. |
| Access to care | Initiation and engagement of alcohol and other drug dependence (AOD) treatment | In HEDIS | Claims | Members 13 years of age and older with a new episode of AOD during the first 10 and ½ months of the measurement year. | · Initiation of AOD Treatment. The percentage of members who initiate treatment through an inpatient AOD admission, outpatient visit, intensive outpatient encounter or partial hospitalization within 14 days of the diagnosis. · Engagement of AOD Treatment. The percentage of members who initiated treatment and who had two or more additional services with a diagnosis of AOD within 30 days of the initiation visit. |
| Coordination | Follow-up after hospitalization for mental illness | In HEDIS | Claims | Members 6 years of age and older who were hospitalized for treatment of mental illness. | · 30-day Follow-up. Discharges for which the member received follow-up within 30 days of discharge. · 7-day Follow-up. Discharges for which the member received follow-up within 7 days of discharge. |
| Follow-up after emergency department (ED) visit for mental illness | In HEDIS | Claims | Members who had an ED visit with a primary mental health diagnosis. | Members who received an outpatient or partial hospitalization visit with a primary diagnosis of mental health. | |
| Follow-up after ED visit for AOD | In HEDIS | Claims | Members who had an ED visit with a primary AOD diagnosis. | Members who received an outpatient or partial hospitalization visit with a primary diagnosis of AOD. | |
| Follow-Up After Emergency Department Visit for People with High-Risk Multiple Chronic Conditions | In HEDIS | Claims | Members who had an ED visit for two or more serious chronic conditions, including depression as well as Alzheimer’s, atrial fibrillation, chronic kidney disease, COPD/asthma, cardiovascular (heart failure, heart attack, stroke) | Percentage of emergency department visits for members who have high-risk multiple chronic conditions and had a follow-up service within 7 days of the ED visit. | |
| “Integration” of medical needs | Diabetes screening for people with schizophrenia or bipolar disorder who are using antipsychotic medications | In HEDIS | Claims | Members 18-64 years of age with schizophrenia or bipolar disorder and dispensed an antipsychotic medication. | Members who had a glucose test or HbA1c test during the measurement year. |
| Diabetes monitoring for people with diabetes and schizophrenia | In HEDIS | Claims | Members 18-64 years of age with schizophrenia and diabetes. | Members who had an HbA1c test and an LDL-C during the measurement year. | |
| Cardiovascular monitoring for people with cardiovascular disease and schizophrenia | In HEDIS | Claims | Members 18–64 years of age with schizophrenia and cardiovascular disease. | Members who had an LDL-C test performed during the measurement year. | |
| Metabolic monitoring for children and adolescent on antipsychotics | In HEDIS | Claims | Members 1–17 years of age who had two or more antipsychotic prescriptions. | Members who had both of the following during the measurement year. · At least 1 blood glucose/HbA1c test. · At least 1 LDL-C or cholesterol test. | |
| Overuse/ Appropriateness | Use of multiple concurrent antipsychotics in children and adolescents | In HEDIS | Claims | Children and adolescents 1–17 years of age who were dispensed an antipsychotic medication. | Members who were on two or more concurrent antipsychotic medications for at least 90 consecutive days during the measurement year. |
| Opioid overuse
| In HEDIS | Claims | Members 18 years of age and older receiving prescription opioids for > 15 days during the measurement year. | · High Dosage. Members who received a daily dosage of opioids greater than 120 mg morphine equivalent dose (MED) for 90 consecutive days or longer. · Multiple Prescribers and Multiple Pharmacies. Members who received prescriptions for opioids from four (4) or more prescribers AND four (4) or more pharmacies. · Multi-Provider, High Dosage: Members who had prescriptions for opioids greater than 120mg morphine equivalent dose (MED) for 90 consecutive days or longer, AND who received opioid prescriptions from four (4) or more prescribers AND four (4) or more pharmacies. | |
| Utilization | Identification of alcohol and other drug services | In HEDIS | Claims | All members | Members who received the following chemical dependency services during the measurement year: · Any service. · Inpatient. · Intensive outpatient or partial hospitalization. · Outpatient or ED. |
| Mental health utilization | In HEDIS | Claims | All members | Members who received the following mental health services during the measurement year: · Any service. · Inpatient. · Intensive outpatient or partial hospitalization. · Outpatient or ED. | |
| Use of first-line psychosocial care for children and adolescent on antipsychotics | In HEDIS | Claims | Children and adolescents 1–17 years of age who had a new prescription for an antipsychotic medication. | Members who had documentation of psychosocial care in the 121-day period from 90 days prior to the IPSD through 30 days after the IPSD. | |
| Outcomes | Depression remission/response
| Under development | ECDS | Member 12 years of age and older with a diagnosis of major depression or dysthymia and an elevated PHQ-9 score. | Members who had evidence of response or remission within 5–7 months of the elevated PHQ-9 score. |
Additional NCQA measures address “integrated care” for behavioral health conditions (not in HEDIS)
| Measure | Data Source | Denominator | Numerator |
| Alcohol Screening and Follow-up for People with Serious Mental Illness (SMI) | Claims | Members 18 years of age and older with at least one inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year. | Members who were screened for unhealthy alcohol use and received two events of counseling if identified as an unhealthy alcohol user. |
| Tobacco Use Screening and Follow-up for People with Serious Mental Illness or Alcohol and Other Drug Dependence | Claims | · SMI: Members 18 years of age and older with at least one inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year. · AOD: All members 18 years of age or older as of December 31 of the measurement year with any diagnosis of alcohol or other drug dependence during the measurement year. | · SMI: Members who were screened for tobacco use and received follow-up care if identified as a current tobacco user. · AOD: Members who were screened for tobacco use and received follow-up care if identified as a current tobacco user. |
| Body Mass Index Screening and Follow-up for People with Serious Mental Illness | Claims | Members 18 years of age and older with at least one inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year. | Members who had calculated body mass index documented and were provided two events of follow-up care if body mass index was greater than or equal to 30 kg/m2. Follow-up includes: · Two events of counseling, on different dates, for weight management (such as nutrition or exercise counseling) or · One event of counseling and one fill of medication (Orlistat) for weight management. |
| Controlling High Blood Pressure for People with Serious Mental Illness | Claims | Members 18-85 years of age with at least one acute inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year AND a diagnosis of hypertension. | Members whose most recent blood pressure (BP) was adequately controlled (after the diagnosis of hypertension) based on the following criteria: · Members 18-59 years of age whose BP was <140/90 mm Hg. · Members 60-85 years of age and flagged with a diagnosis of diabetes whose BP was <140/90 mm Hg. · Members 60-85 years of age and flagged as not having a diagnosis of diabetes whose BP was <150/90 mm Hg. |
| Comprehensive Diabetes Care for People with Serious Mental Illness: Hemoglobin A1c (HbA1c) Testing | Claims | Members 18-75 years of age with at least one acute inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year AND diabetes (type 1 and type 2). | Members who had an HbA1c test performed. |
| Comprehensive Diabetes Care for People with Serious Mental Illness: Hemoglobin A1c (HbA1c) Poor Control (>9.0%) | Claims | Members 18-75 years of age with at least one acute inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year AND diabetes (type 1 and type 2). | Members whose most recent HbA1c level was greater than 9.0% or was missing a result, or for whom an HbA1c test was not done. |
| Comprehensive Diabetes Care for People with Serious Mental Illness: Medical Attention to Nephropathy | Claims | Members 18-75 years of age with at least one acute inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year AND diabetes (type 1 and type 2). | Members who received a nephropathy screening test or had evidence of nephropathy. |
| Comprehensive Diabetes Care for People with Serious Mental Illness: Blood Pressure Control (<140/90 mm Hg) | Claims | Members 18-75 years of age with at least one acute inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year AND diabetes (type 1 and type 2). | Members whose most recent blood pressure screening result was <140/90mm Hg. |
| Comprehensive Diabetes Care for People with Serious Mental Illness: Hemoglobin A1c (HbA1c) Control (<8.0%) | Claims | Members 18-75 years of age with at least one acute inpatient visit or two outpatient visits for schizophrenia or bipolar I disorder, or at least one inpatient visit for major depression during the measurement year AND diabetes (type 1 and type 2). | Members whose most recent HbA1c level was less than 8.0%. |
| Comprehensive Diabetes Care for People with Serious Mental Illness: Eye Exam | Claims | Members 18-75 with at least 1 acute inpatient visit or 2 outpatient visits for schizophrenia or bipolar disorder, or at least 1 inpatient visit for major depression during the measurement year AND diabetes (type 1 and type 2). | Members who received an eye exam. |
[1] HEDIS – the Healthcare Effectiveness and Data Information Set, is a registered trademark of NCQA.
[2] Please see Appendix A for a full list of HEDIS behavioral and related measures.
[3] NCQA Comments on CMS Request for Information on Data Differences in Medicare Advantage and Part D Star Rating Quality Measurements for Dual-eligible vs. Non-dual Eligible Enrollees, November 3, 2014.
[4] NCQA-Accredited Medicare Advantage Plans Perform Better Fact Sheet, NCQA, 2017.
