HEDIS Electronic Clinical Data Systems (ECDS) Reporting
Our newest reporting method helps clinical data create insight for managing the health of individuals and groups.
ECDS News
Measure Updates
| New ECDS Measures | Tobacco Use Screening and Cessation Intervention (TSC-E)
Follow-Up After Acute Care Visits for Asthma (AAF-E) Blood Pressure Control for Patients With Diabetes (BPD-E) |
| Transition to ECDS | Lead Screening in Children (LSC-E)
Therapy for Patients With Diabetes (SPD-E) Statin Therapy for Patients With Cardiovascular Disease (SPC-E) |
Removal of the SSoR Reporting Requirement
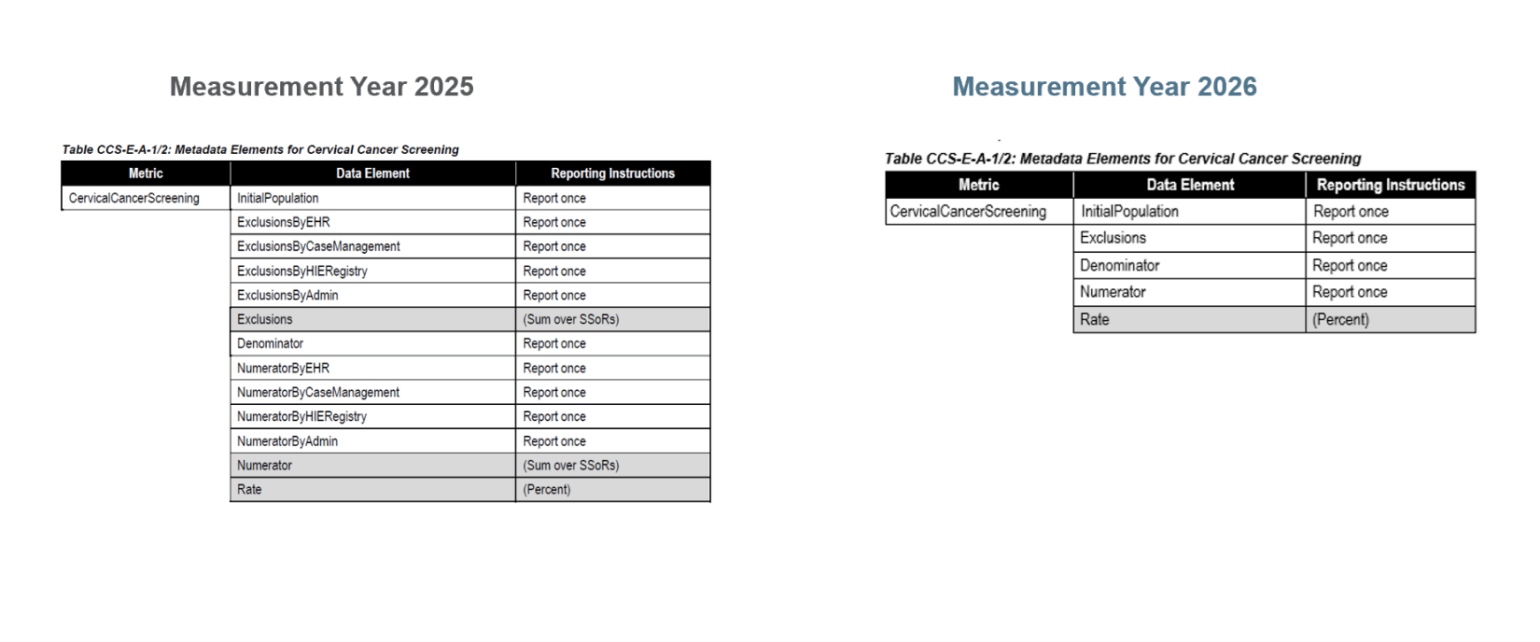
As of Measurement Year (MY) 2026, source system of record (SSoR) reporting will no longer be required for ECDS reporting.
The SSoR data source contains elements used in HEDIS reporting. For ECDS reporting, health plans must submit HEDIS measure data by each SSoR accessed to produce the measure result.
There are four SSoR reporting categories:
- Electronic health record (EHR)/personal health record (PHR).
- Health information exchange (HIE)/clinical registry.
- Case management registry.
- Administrative.
SSoR reporting identifies the origin of data used in quality measurement, with the intent of improving understanding of ECDS reporting trends. HEDIS reporting data have shown variation in use and contribution of clinical data sources to reporting over time.
As NCQA grows the measures available for ECDS reporting, we are removing this requirement to help simplify HEDIS reporting and enable the transition to digital quality measurement. We are also exploring other methods to evaluate the use of data sources, and establish data provenance in alignment with interoperability standards.
For MY 2026, NCQA removed the SSoR reporting data elements from the data elements tables for all ECDS-reported measures (example below).
Helping States Move Towards a Digital Quality System
As health care systems evolve to support greater interoperability of electronic health care information, quality measurement must also evolve. This resource guide outlines considerations and opportunities for state regulators supporting the move toward the digital quality system of tomorrow.
Issue Brief on Leveraging Electronic Clinical Data for HEDIS®
In 2019 and 2020, NCQA interviewed health plans that reported HEDIS using the ECDS reporting standard. This issue brief showcases strategies and opportunities to advance the collection and use of clinical data for improving care and quality measurement.
Reporting Results for Measures Leveraging Electronic Clinical Data
NCQA has published special reports summarizing HEDIS results for measures that use the Electronic Clinical Data Systems reporting standard. These reports, published in 2021, 2022, 2024 and 2025, highlight reporting trends over time as well as performance results across the measures.
Resource Guide on Leveraging Clinical Data for Measurement of Colorectal Cancer Screening
This resource guide supports health plans and other stakeholders in successfully reporting measures using electronic clinical data systems—specifically, on using electronic clinical data in reporting the HEDIS measure Colorectal Cancer Screening. The strategies and resources outlined in this guide can be adapted to other quality measures and improvement use cases.
NCQA announced public reporting for the following HEDIS measures using the ECDS reporting standard. For information about use of these measures in programs such as NCQA’s Health Plan Ratings, refer to our FAQ page.
Measurement Year 2020
- Prenatal Immunization Status (PRS-E).
Measurement Year 2022
- Adult Immunization Status (AIS-E).
- Breast Cancer Screening (BCS-E).
- Prenatal Depression Screening and Follow-Up (PND-E).
- Postpartum Depression Screening and Follow-Up (PDS-E).
Measurement Year 2023
- Depression Remission or Response for Adolescents and Adults (DRR-E).
- Depression Screening and Follow-Up for Adolescents and Adults (DSF-E)
- Follow-Up Care for Children Prescribed ADHD Medication (ADD-E).
- Metabolic Monitoring for Children and Adolescents on Antipsychotics (APM-E).
- Unhealthy Alcohol Use Screening and Follow-Up (ASF-E).
- Utilization of the PHQ-9 to Monitor Depression Symptoms for Adolescents and Adults (DMS-E).
Measurement Year 2024
- Colorectal Cancer Screening (COL-E).
- Note: In HEDIS MY 2019, NCQA added the ECDS reporting standard to the Colorectal Cancer Screening (COL) measure for voluntary reporting, alongside traditional reporting. This allowed health plans to assess their ECDS reporting capabilities and compare performance using both methods. Reporting results and stakeholder feedback, including that of NCQA’s Committee on Performance Measurement (CPM) and Standards Committee, supported transitioning the COL measure to ECDS-only reporting in MY 2024. COL-E results will be publicly reported in 2025 and used in 2025 Health Plan Ratings. NCQA has released MY 2023 national averages by product line for the COL-E measure at our State of Health Care Quality Report.
- Cervical Cancer Screening (CCS-E).
- Childhood Immunization Status (CIS-E).
- Immunizations for Adolescents (IMA-E).
NCQA added the ECDS reporting standard to seven existing HEDIS measures for voluntary reporting alongside their traditional counterparts. This allows health plans to assess their ECDS reporting capabilities using familiar measures. Based on reporting results to date and stakeholder feedback, NCQA has announced the transition of these measures to ECDS-only reporting for the following years:
Measurement Year 2023:
- Breast Cancer Screening (see this blog for more information).
Measurement Year 2024:
- Colorectal Cancer Screening (see this blog for more information).
- Follow-Up Care for Children Prescribed ADHD Medication.
- Metabolic Monitoring for Children and Adolescents on Antipsychotics.
Measurement Year 2025:
- Childhood Immunization Status.
- Immunizations for Adolescents.
- Cervical Cancer Screening.
Measurement Year 2026:
- Lead Screening in Children.
- Statin Therapy for Patients With Diabetes.
- Statin Therapy for Patients With Cardiovascular Disease.
NCQA continues to explore how electronic clinical data can be leveraged in more HEDIS quality measures in the future.
ECDS In Brief
The ECDS reporting standard gives health plans a method for collecting and reporting standard electronic clinical data for HEDIS quality measurement and improvement.
The ECDS architecture was designed to help HEDIS implementers understand how technology might improve the efficiency of quality reporting while providing an incentive to connect to a broad array of actionable information from multiple sources.
HEDIS quality measures reported using ECDS inspire innovative use of electronic clinical data to document high-quality patient care.
The ECDS reporting standard represents a step forward in the evolution of HEDIS to accommodate the extensive information available in electronic datasets used for patient care and quality improvement.
Measures that use HEDIS ECDS Reporting Method
Measurement Year 2026
Behavioral health
- Depression Screening and Follow-Up for Adolescents and Adults
- Utilization of the PHQ-9 to Monitor Depression Symptoms for Adolescents and Adults
- Depression Remission or Response for Adolescents and Adults
- Unhealthy Alcohol Use Screening and Follow-Up
- Prenatal Depression Screening and Follow-Up
- Postpartum Depression Screening and Follow-Up
- Follow-up Care for Children Prescribed ADHD Medication
- Metabolic Monitoring For Children and Adolescents on Antipsychotics
Preventive screening
- Breast Cancer Screening
- Colorectal Cancer Screening
- Cervical Cancer Screening
- Documented BI-RADS Assessment Mammogram
- Follow-Up After Abnormal Mammogram Assessment
- Lead Screening in Children
Health equity
- Social Needs Screening and Intervention
Immunizations
- Prenatal Immunization Status
- Adult Immunization Status
- Childhood Immunization Status
- Immunization for Adolescents
Management of chronic conditions
- Blood Pressure Control for Patients with Hypertension
- Follow-Up After Acute Care Visits for Asthma
- Statin Therapy for Patients with Diabetes
- Statin Therapy for Patients with Cardiovascular Disease
- Blood Pressure Control for Patients with Diabetes
Types of ECDS Data
Data systems that may be eligible for HEDIS ECDS reporting include, but are not limited to, member eligibility files, EHRs, clinical registries, HIEs, administrative claims systems, electronic laboratory reports (ELR), electronic pharmacy systems, immunization information systems (IIS) and disease/case management registries.
Data sources used for HEDIS ECDS reporting are categorized as follows:
- Electronic health record (EHR)/personal health record (PHR). Patient records that document a patient’s medical history, treatment plans, radiology and laboratory test results in digital format that can make the information available instantly and securely to authorized users.
- Health information exchange (HIE)/clinical registry. HIEs and clinical registries eligible for this reporting category include state HIEs, IIS, public health agency systems, regional HIEs (RHIO), Patient-Centered Data Homes™ or other registries developed for research or to support quality improvement and patient safety initiatives. Clinical registries may be sponsored by a government agency, nonprofit organization, health care facility or private company, and decisions regarding use of a registry’s data are the responsibility of the registry’s governing committee.4
- Case management system. A shared database of member information collected through a collaborative process of member assessment, care planning, care coordination or monitoring of a member’s functional status and care experience. Case management systems eligible for this category of ECDS reporting include any system developed to support the organization’s case/disease management activities, including activities performed by delegates.
- Administrative. Includes data from administrative claim processing systems for all services incurred (paid, suspended, pending, denied) during the period defined by each measure’s participation, as well as member management files, member eligibility and enrollment files, electronic member rosters, internal audit files and member call service databases.
Submit questions about HEDIS ECDS reporting through My NCQA.
ECDS Frequently Asked Questions
See FAQs here.
- HEDIS® is a registered trademark of the National Committee for Quality Assurance (NCQA).
- https://www.federalregister.gov/documents/2015/10/16/2015-25597/2015-edition-health-information-technology-health-it-certification-criteria-2015-edition-base
- https://www.healthit.gov/providers-professionals/health-information-exchange/what-hie
- https://www.nih.gov/health-information/nih-clinical-research-trials-you/list-registries
Understanding ECDS Measures & the Hybrid Phase out
Get familiar with understanding how ECDS measures support the Digital Transition
The Transformation of HEDIS
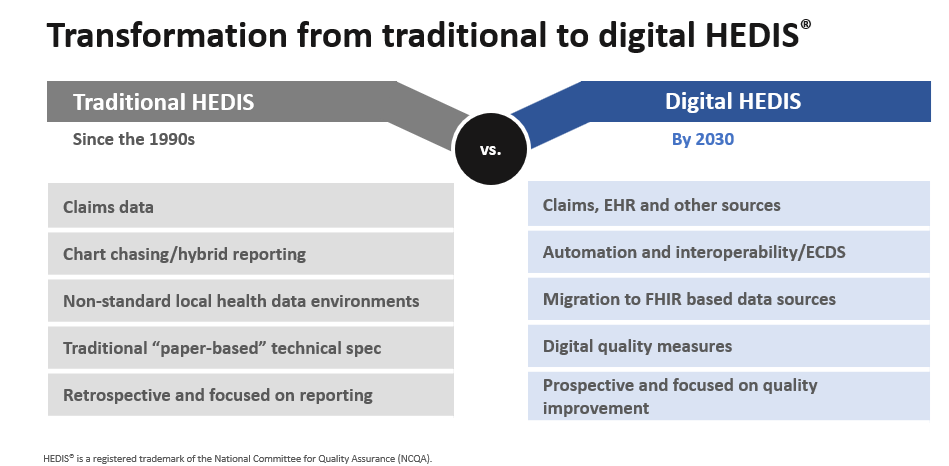
NCQA’s vision for the future of measures is the use of digital quality measures (dQMs). The transition involves a multi-phase approach towards fully automated, interoperable measurement systems. The goal is to eliminate manual processes, enable real-time insights, and align data collection with clinical workflows to reduce administrative burden.
Electronic Clinical Data Systems (ECDS) and Digital Quality Measures (dQM) are related but not synonymous.
Electronic Clinical Data Systems (ECDS)
- A HEDIS® reporting standard for health plans that collect and submit quality measures
- A structured way to aggregate electronic data for HEDIS reports
- Supports electronic exchange of clinical data
Digital Quality Measure (dQM)
- A standard, interoperable format
- Computer interpretable, fully specified, standards-based (i.e., FHIR-CQL) measure content
- Improves the delivery of specifications and makes it easier to use measure content
- ECDS and traditional HEDIS measures are available as dQMs through Digital Content Services.
Visit the Digital Quality Hub to learn more about NCQA’s Digital Quality Transition.
Hybrid Transition
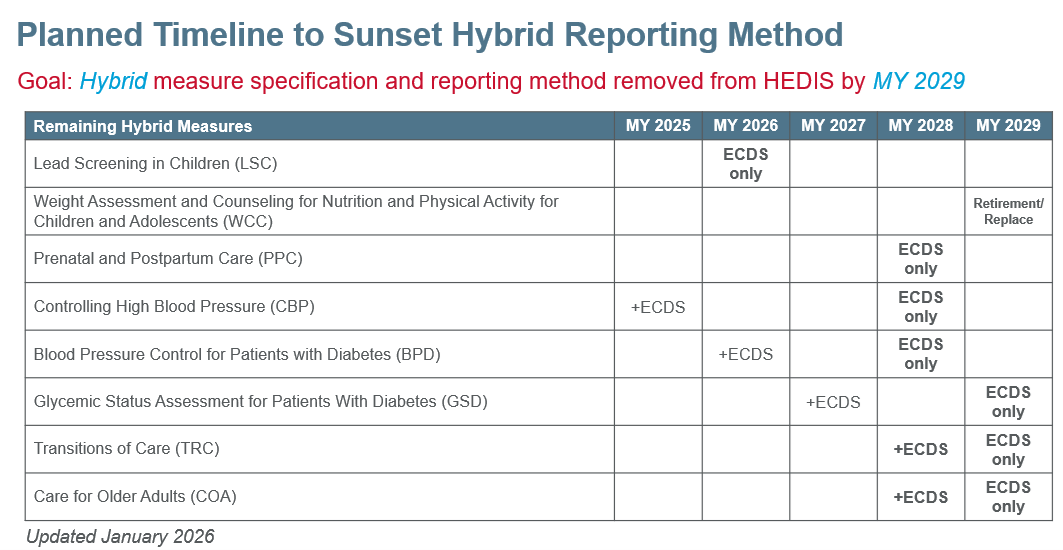
The shift to digital measurement involves phasing out the Hybrid reporting method, easing the burden of manual record retrieval, and advancing towards making quality measures available as FHIR/CQL computable dQMs. As of MY2025, eight HEDIS measures still allowed Hybrid reporting. In 2024, NCQA announced a planned transition to remove the Hybrid method by MY 2029. Some measures will transition directly to ECDS while others will introduce an ECDS version for optional reporting during a transition period before the Hybrid specification is fully retired.
While the MY 2029 endpoint remains unchanged, in January 2026, NCQA refined certain measure-specific pathways and interim timelines due to realignment efforts and the need for additional measure testing.
Overview of Changes:
- Weight Assessment and Counseling for Nutrition and Physical Activity for Children/Adolescents: Instead of transitioning to administrative-only reporting in MY 2027, NCQA is prioritizing measure retirement in MY 2029. In parallel, NCQA intends to develop a replacement measure.
- Prenatal and Postpartum Care: Instead of moving to administrative-only reporting in MY 2028, NCQA is focusing on the development of a new ECDS and risk-based replacement measure by MY 2028, with retirement of the Hybrid version occurring concurrently.
- Transitions of Care and Care for Older Adults: NCQA will delay introducing the new ECDS versions until MY 2028. Both measures will be optionally reported until the Hybrid method is removed in MY 2029.
See the current hybrid transition timeline below.
Replacing the Hybrid measures has been a deliberate process. It starts with developing and testing the new ECDS measure, including parallel testing with the existing hybrid measure to compare results and ensure accuracy. Once validated, the new ECDS measure becomes part of the official HEDIS reporting, integrating into health plan workflows. The final step is retiring the original hybrid measure after successful implementation, fully transitioning to digital reporting to reduce manual processes and enhance efficiency.
During the transition, NCQA evaluates HEDIS data to confirm the ECDS measure meets quality standards, provides benchmark guidance for health plans, and collaborates with standard organizations to address digital feasibility gaps. This phased approach ensures thorough testing, validation, and stakeholder engagement, maintaining data integrity and reporting accuracy throughout.
Visual Pathway for Replacing Hybrid Measures with ECDS:
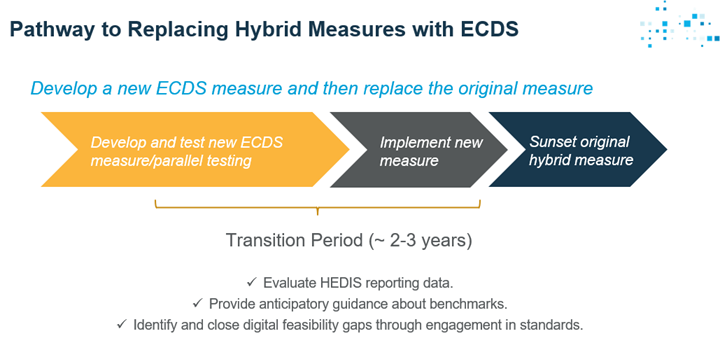
- Develop the new ECDS measure, implement it, then sunset the original measure.
- Both old and new measures will be in HEDIS simultaneously.
This transition period enables us to:
- Compare performance between original and new measures.
- Provide anticipatory guidance about benchmarks.
- Identify and close digital feasibility gaps through standards engagement.
Understand the Difference in the Reporting Methods in HEDIS
There are some key highlights that are different in the different reporting methods.
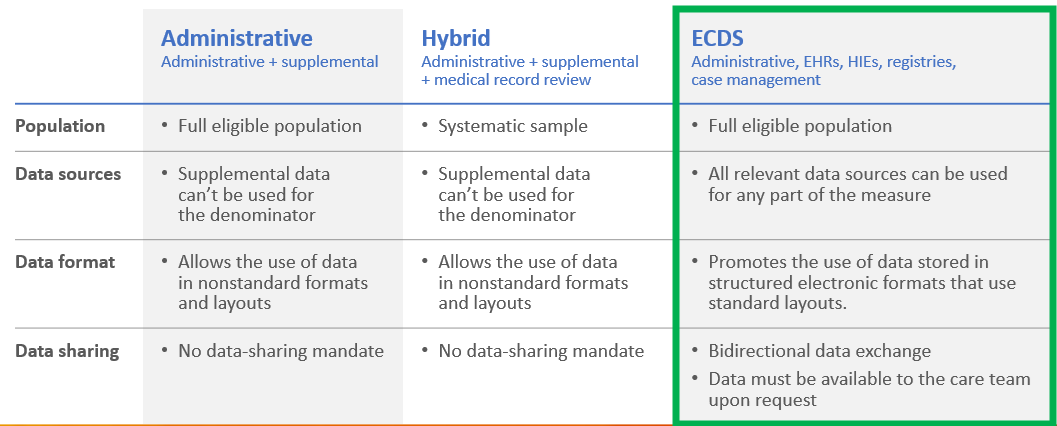
HEDIS ECDS Data Collection Considerations
Transition to ECDS Reporting